| AIR POLLUTION IN THE CZECH REPUBLIC IN 2012 Czech Hydrometeorological Institute - Air Quality Protection Division |
|
|
|
|
|
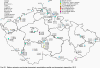
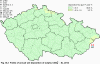
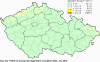
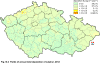
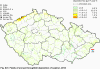
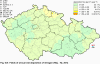
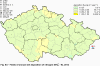
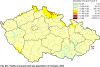
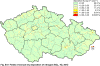
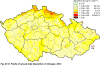
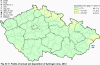
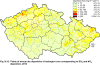
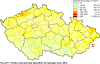
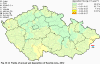
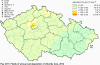
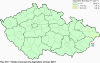
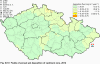
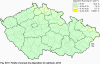
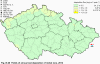
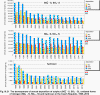
III. ATMOSPHERIC DEPOSITION IN THE CZECH REPUBLIC Chemical composition (precipitation quality) and atmospheric deposition have been monitored in the long term at relatively large number of stations in the Czech Republic. In 2012 the Air Quality Information System (ISKO/AQIS) database obtained data on precipitation quality from 44 localities in total (13 ÄŚGS, 14 CHMI, 8 VĂšLHM, 1 VĂšV TGM, 6 HBĂš AV ÄŚR and 2 KRNAP, see Fig. III.1). Further, data from 5 German (by LfuG) and 3 Polish localities (by WIOS) in boundary areas were submitted. Most of the CHMI stations measure wet-only samples in weekly interval (monthly interval was switched over to weekly interval in 1996 in line with the EMEP methodology). Further, from 1997 to 2010 the weekly precipitation sampling, “bulk” type (with non-specified content of dustfall), for heavy metals analysis was carried out at these stations. Since 2011 the analyses of heavy metals at CHMI stations have been carried out from wet-only precipitation sampling, “bulk” type sampling was closed down. In the localities of other organizations monthly sampling (or irregular sampling) is used for measuring concentrations in precipitation (“bulk” type) in the open area (or throughfall). The detailed information on individual localities and sampling types is presented in Table III.4. Wet deposition charts were compiled for selected ions on the basis of all-round chemical analyses of wet only precipitation samples, specifically for SO42-- S, NO3-- N, NH4+- N, H+ (pH), F-, Pb2+, Cd2+ and Ni2+ . The above ions were selected to represent deposition fields with regard to their considerable impact on the various spheres of the environment. Wet deposition charts for each of the ions were derived from the field of ion concentrations in precipitation (based on annual mean concentrations weighted by precipitation totals calculated from the data observed), and from the field of annual precipitation totals which was generated on data from 750 precipitation gauging stations, taking into account the altitude’s effect on precipitation amount. When constructing wet deposition fields, results of wet-only samples analysis are preferred to “bulk” samples with dustfall, and weekly samples are preferred to monthly samples. Data from the network stations operated by ÄŚGS, VĂšV, VĂšLHM and KRNAP based on monthly “bulk” sampling with dustfall (see Table III.4) are modified by empirical coefficients expressing the individual ions’ ratios in “wet-only” and “bulk” samples (values for each of the ions from 0.74 for NH4+ to 1.06 for H+) for the purpose of the development of the wet deposition charts. The fact that in case of H+ cations the ratio is higher than 1, can be explained in the following way: the solid particles contained in the “bulk” type samples react with hydrogen cations, which results in their decreasing concentration [48]. In addition to wet deposition, also dry deposition charts are presented for sulphur, nitrogen, hydrogen ions, lead and cadmium. The maps of total annual deposition are presented for sulphur, nitrogen and hydrogen ions. Dry sulphur and nitrogen deposition was calculated using fields of annual mean SO2 and NOx concentrations for the Czech Republic, and the deposition rates for SO2 0.7 cm.s-1/0.35 cm.s-1, and NOx 0.4 cm.s-1 / 0.1 cm.s-1, for the forested/unforested areas [49]. Total deposition charts were produced by adding S and N wet and dry deposition charts. The wet hydrogen ion deposition chart was compiled on the base of pH values measured in precipitation. Dry hydrogen ion deposition reflects SO2 and NOx deposition based on stechiometry, assuming their acid reaction in the environment. The total hydrogen ion deposition chart was developed by summation of wet and dry deposition charts. The average deposition fluxes of S, N and H are presented in
the Table III.1. Throughfall sulphur deposition chart was generated for forested areas from the field of sulphur concentrations in throughfall and a verified field of precipitation, which was modified by a percentage of precipitation amounts measured under canopy at each station (61–90 % of precipitation totals in an open area for the year 2012). Throughfall deposition generally includes wet vertical and horizontal deposition (from fogs, low clouds and rime) and dry deposition of particles and gases in forests. In case of sulphur, its circulation within the forests is negligible; it should provide a good estimate of total deposition. The fields of dry deposition of Pb and Cd contained in SPM (dry Pb and Cd deposition) were derived from the fields of these metals’ concentrations in the ambient air (or on the basis of air pollution field of annual average of PM10 concentrations and values of IDW interpolation of the shares of the respective metal in dust). The deposition rate of Cd contained in SPM was taken as 0.27 cm.s-1 for a forest and 0.1 cm.s-1 for unforested terrain; the figures for Pb are 0.25 cm.s-1 for a forest and 0.08 cm.s-1 for unforested terrain [49]. The data on precipitation quality are controlled routinely using the method of ion balance calculation. The difference between the sum of cations and the sum of anions in the sample should meet the allowable criteria which differ slightly in various organizations. Another control is carried out by comparing the calculated conductivity and the measured conductivity which both should also meet the allowable criteria. Analysis of the blank laboratory samples is also used and blank field samples are monitored and assessed continuously. This enables the control of work during sampling and the control of changes occurring due to transport, manipulation, storage and preparation of the samples prior to the chemical analysis. Results
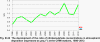
The development of annual wet deposition of the main elements as measured at selected stations in the Czech Republic (Fig. III.23) after the decrease of wet deposition of several components (mainly sulphates, hydrogen ions and lead ions) in the second half of the 90’s, shows stagnation instead. The decrease of sulphate deposition was apparent both at the relatively exposed suburban stations and at the background stations, e.g. Košetice and Svratouch. With the development of sulphur and nitrogen deposition the development of the proportion of both elements can be observed in atmospheric precipitation connected with the development of emissions of individual pollutants. Since the second half of the 90’s a slight increase of nitrates and sulphates proportion has been observed at some stations The development of this proportion over the recent 15 years for the CHMI stations is shown in Fig. III.22. Tab. III.1 Average deposition fluxes of S, N and H in the Czech Republic, 2012 Tab. III.2 Estimate of the total annual deposition of the given elements on the area of the Czech Republic (78,841 sq. km) in tonnes, 2012 Tab. III.3 Estimate of the total annual deposition of sulphur on the forested part of the Czech Republic (26,428 sq. km) in tonnes, 2001–2012 Tab. III.4 Station networks monitoring atmospheric precipitation quality and atmospheric deposition, 2012
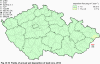
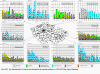
Fig. III.1 Station networks monitoring atmospheric precipitation quality and atmospheric deposition, 2012 Fig. III.2 Fields of annual wet deposition of sulphur (SO42-–S), 2012 Fig. III.3 Fields of annual dry deposition of sulphur (SO2–S), 2012 Fig. III.4 Fields of annual total deposition of sulphur, 2012 Fig. III.5 Fields of annual throughfall deposition of sulphur, 2012 Fig. III.6 Fields of annual wet deposition of nitrogen (NO3-–N), 2012 Fig. III.7 Fields of annual wet deposition of nitrogen (NH4+–N), 2012 Fig. III.8 Fields of annual total wet deposition of nitrogen, 2012 Fig. III.9 Fields of annual dry deposition of nitrogen (NOx–N), 2012 Fig. III.10 Fields of annual total deposition of nitrogen, 2012 Fig. III.11 Fields of annual wet deposition of hydrogen ions, 2012 Fig. III.12 Fields of annual dry deposition of hydrogen ions corresponding to SO2 and NOx deposition, 2012 Fig. III.13 Fields of annual total deposition of hydrogen ions, 2012 Fig. III.14 Fields of annual wet deposition of fluoride ions, 2012 Fig. III.15 Fields of annual wet deposition of chloride ions, 2012 Fig. III.16 Fields of annual wet deposition of lead ions, 2012 Fig. III.17 Fields of annual dry deposition of lead, 2012 Fig. III.18 Fields of annual wet deposition of cadmium ions, 2012 Fig. III.19 Fields of annual dry deposition of cadmium, 2012 Fig. III.20 Fields of annual wet deposition of nickel ions, 2012 Fig. III.21 The development of annual deposition of sulphur (SO42-–S, SO2–S) and oxidated forms of nitrogen (NO3-–N, NOx–N) and hydrogen in the Czech Republic, 1995–2012 Fig. III.22 The development of the ratio of nitrate/sulphate concentrations in atmospheric deposition (expressed as μeq. l-1) at the CHMI stations, 1998–2012 Fig. III.23 The development of annual wet deposition at selected stations in 1991–2012, Czech Republic
|